Our Research
ORGANIC SYNTHESIS AND BIOMIMICRY
The Feng Group will focus on the research on the design and synthesis of dynamic functional molecules and materials that mimic molecular behavior in organisms. We start with small molecules, demonstrate their properties and then use them as tools for the exploration of materials and biomimicry in collaboration with physicists, biologists and chemical engineers. The scientific questions that our group is interested in answering are how we can learn from biomolecules to achieve those fantastic life-like functions in the nano-world in addition to discovering the origin of homochirality, self-replication, autonomous motion, structural transformation and ultimately — life.
Research Key Words
Reaction | Replication | Synthesis | Catalysis | Homochirality | Self-Assembly
Nanoscopic Transformation | Unidirectional Motion | Host–Guest Interaction
Electron Transfer | Dynamical Materials | Molecular Machines | Origin of Life
Dynamic Materials with Nanoscopic Transformations
Mechanically interlocked architectures — which contain topologically complex building blocks (monomers) — will exhibit extraordinary properties on account of new degrees of freedom. While maintaining the same topology, changing the relative relationship between monomers will transform the material significantly. To make dynamic materials at the macroscale, switchable monomers at the nanoscale are required, since a small change will make a big difference. Our current project applies topologically complex and controllable structures to construct materials in order to fulfill dynamic functions.






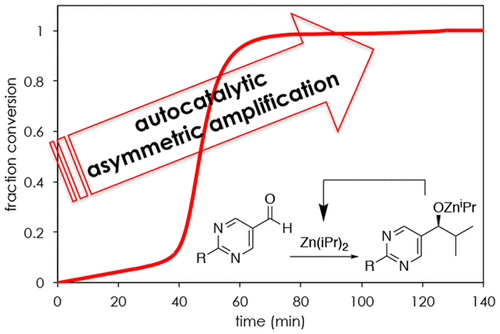
Template-Directed Stereoselective Autocatalysis
Classic stereoselective reactions require chiral ligands or the introduction of a chiral center. Autocatalytic reactions, however, produce stereoisomerically enriched products from achiral precursors. Exploring the origin of chiral amplification can be used for asymmetric synthesis. Introducing recognition sites to reactants can bring templation to the asymmetric bond formation, which will boost the stereoselectivity of desired products. Our project also discovers the origin of homochirality in biomolecules and the chiral amplification in the prebiotic synthesis.





Biomolecular-Driven Molecular Machines in Water
To apply artificial molecular machines for medical applications, they need to be capable of operating sustainably in water. Our project explores chemical fuels and their waste products that are biocompatible and environmentally friendly and integrate molecular machines with biomolecules to operate in a biomimicking environment. The biological fuels that drive the motor could be chosen from coenzymes that are also involved in cellular respiration. The molecular machines apply presumably for drug delivery for cancer diagnosis and therapy.





Precise Synthesis of Non-Equilibrium Rotaxanes via Pumping in Water
Wu, G*; Park, J.; Liu, W.-G.; Jiao, Y.; Zhang, L.; Han, H.; Tang, C.; Jang, T.; Kim, M. M.; Song, B.; Li, X.; Zhang, R.; Liu, E.; Wu, H.; Wu, Y.; Zhao, X.; Feng, Y.; Li, Q.; Kim, H.; Astumian, R. D.*; Goddard III, W. A.*; Stoddart, J. F.*
Chem 2026, 12, 102878.
Functional Molecular Fluorescent Dyes
Push–pull fluorescent dyes — which feature electron-donating (D) substituents connected to electron-accepting (A) substituents — have been investigated as environmentally sensitive fluorophores. The design and synthesis of fluorescent organic compounds containing push–pull chromophores have emerged as an active area of research over the past two decades, as a result of their potential applications in the fields of photovoltaics, non-linear optics, organic light emitting diodes (OLEDs), fluorescent sensors, and bioimaging. Our project investigates the design and synthesis of new building blocks of fluorescent dyes which leads to dynamic properties based on the supramolecular interactions. The dyes are useful for exploring the fundamental principles of intermolecular and intramolecular electron transfer with the collaboration of optical physicists and theoretical chemists.

Redox Switchable Macrocycles and Cages
Synthetic macrocycles and cages capable of undergoing allosteric regulation by responding to versatile external stimuli are the subject of increasing attention in supramolecular science. Geometries of macrocycles and cages in relation to size, shape, electronic properties, and binding affinities toward polycyclic aromatic hydrocarbons can be readily regulated.
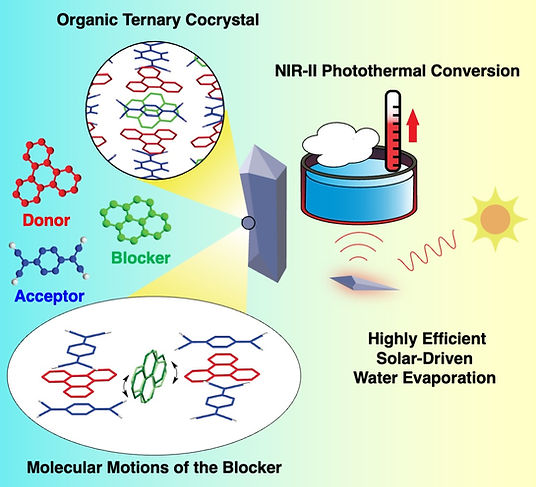
Photothermal Conversion Organic Cocrystals
On account of the scarcity of molecules with a satisfactory second near-infrared (NIR-II) response, the design of high-performance organic NIR photothermal materials has been limited. Organic photothermal materials have been discovered as some of the most promising photothermal agents with economical cost, improved biocompatibility, and potential biodegradability. Cocrystals are obtained typically by the self-assembly of two alternative molecular building blocks, i.e., an electron-rich donor (D) and an electron-poor acceptor (A). Attributed to the adjustable energy gaps, solvent processability, and easy recyclability as a type of crystalline materials, cocrystals present bright prospects in the fields of field-effect transistors, ferroelectricity materials, dynamic photomechanics, and nonlinear optics. Because the energy absorption and transfer can be adjusted through changing their constituting components, cocrystals have been applied in photothermal conversion.






%20TOC.jpg)
![A Donor–Acceptor [2]Catenane for Visible Light Photocatalysis.](https://static.wixstatic.com/media/51a59d_84b9035ed6144df1a1837d1229f20f64~mv2.jpeg/v1/fill/w_460,h_203,al_c,q_80,usm_0.66_1.00_0.01,enc_avif,quality_auto/(17)%20TOC.jpeg)
%20TOC.jpg)
%20TOC.jpg)
%20TOC.jpeg)
%20TOC.png)
%20TOC.jpg)
%20TOC.jpg)
%20TOC.jpg)
%20TOC.jpg)
%20TOC.jpg)
%20TOC.jpg)
%20TOC.jpg)
%20TOC.jpg)
%20TOC.jpg)